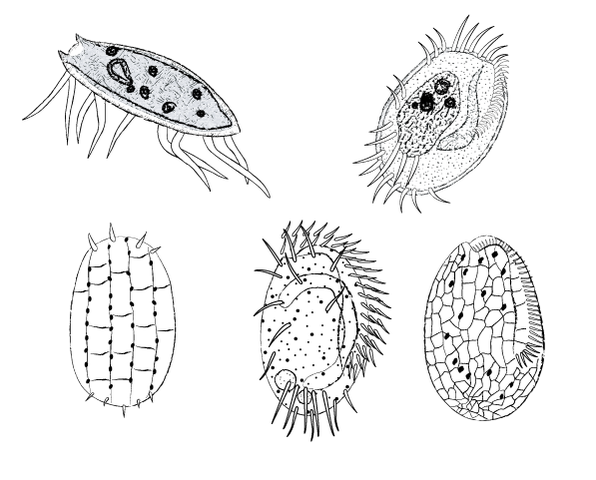
 Free swimming and crawling ciliates common in healthy wastewater systems
Free swimming and crawling ciliates common in healthy wastewater systems - Note floc size, density, and filaments - compare to "normal" system levels (100x)
- Free bacteria and pin floc (best seen at 400x)
- Now look for indicator protozoa (amoeba, flagellates, ciliates, and stalk ciliates)
- Any higher life forms such as rotifers or worms
- Finally, anything unusual or big changes from previous exam



 RSS Feed
RSS Feed