Why run the test for 30 minutes - why not an hour or two which is more like the clarifier?
Unlike the clarifier, the SV30 settleometer or cylinder does not have flow or in most cases stirring with a rake. You are looking for three distinct things in the test:
- How fast d.oes the MLSS settle - readings at 5 min intervals can be helpful in problem systems
- How much pinfloc, fines, or turbidity is in the supernatant
- How well does the sludge compact at the bottom - good prediction for clarifier bed depth & RAS concentration
If we go beyond 30 minutes, we are looking more at sludge compaction. Since there is not stirring or flow, denitrification can start to compromise test results - although it can help explain floating solids in the clarifier and need to increase recycle rates. (MLSS in clarifier beds held too long can denitrify and float like DAF solids.
Is there an advantage to plotting the settling/compaction curve at 5 minute intervals?
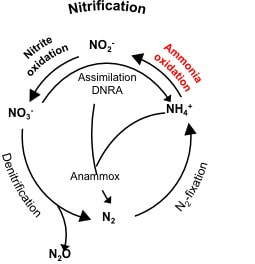
In a one word answer, YES. More data helps troubleshoot and predict when the system is starting to have settling problems. Plotting the settling rates gives insight into filamentous and non-filamentous bulking, "old sludge", and can potentially inform you of spills, shock loadings. Remember, settling and TSS removal is the first thing to be affected during spills - followed of course by ammonia oxidation/removal.



 RSS Feed
RSS Feed