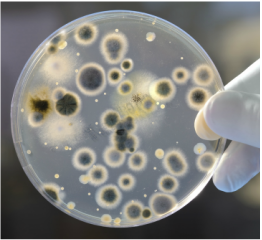
- Isolate candidate microbes from environmental isolates, waste samples, or culture collections.
- Screen to ensure the candidates are non-pathogenic and classified as Bio Safety Level 1 organisms.
- Determine range of environmental conditions in which the strains grow. For example - pH, temperature, anaerobic growth, and nitrate/nitrite utilization.
- Growth on target organics and production of beneficial metabolites – this is where we see if it degrades problem compounds and forms desirable biofilm/floc. We aim to select the strains with best degradation capabilities and shortest-doubling time.
- Investigate the strain ability to be grown in industrial fermentation processes.
- Can the strain be preserved in shelf-stable forms. And conduct long term storage shelf life testing.
- Testing to ensure compatibility with other strains in the consortia.
- Lab testing on waste samples and compare growth to existing strains.
- Field testing to ensure lab results holdup in the real world environment.
- Introduce the new product.
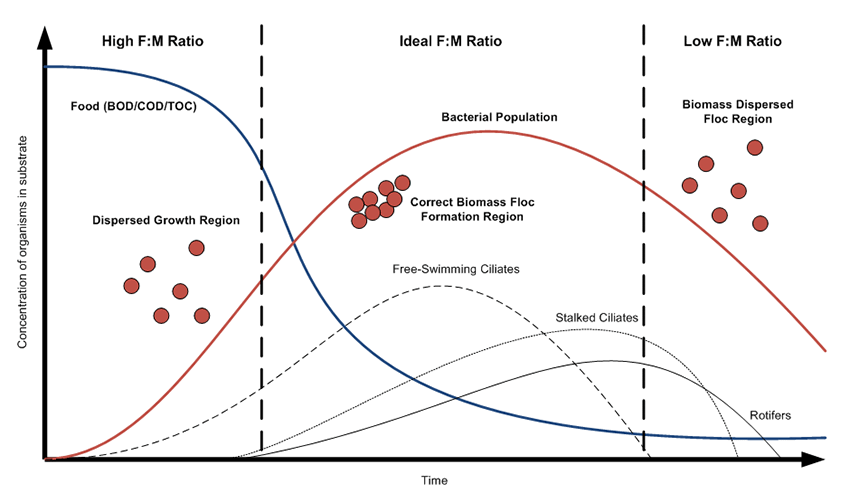

 RSS Feed
RSS Feed