- MLSS & TSS are the same thing. Both measure total solids in a liquid sample. TSS usually refers to effluent solids, where MLSS is usually solids in the aeration basin.
- First weigh the filter to get baseline weight. Make sure the filter is dry!
- MLSS is the total solids. What we do is dry in an oven at 105 Deg C for at least 1 hour - this evaporates the water in the sample leaving dried contents of the sample.
- After calculating the MLSS, you put the same filter in the 550 Deg C furnace for 30 minutes. At 550 Deg C, you flash off the volatile organic fraction leaving inorganic portion of the MLSS. When you weigh the cooled filter, you obtain the weight of non-volatile solids.
- For calculating the volatile solids or MLVSS, you take the MLSS number - the non-volatile solids (last step). This gives you the MLVSS.
- When calculating F/M you can use either MLSS or MLVSS - just make sure you are consistent.
|
I have received several questions on MLSS & MLVSS testing and how to calculate volatiles vs non-volatile fractions. Instead of regurgitating formulas, I will walk through the test calculations the way I remember the math.
Many of us in industrial wastewater have experienced a toxic shock event. In most cases, toxic shock is noted by loss of nitrification and deflocculation (turbidity/floating solids). Today, I will work thorough my process for identifying what caused or is causing the upset.
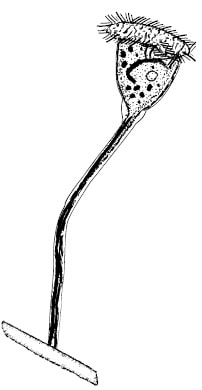 Stalk Ciliate - an ideal protozoa indicator Stalk Ciliate - an ideal protozoa indicator I like it when a facility looks under the microscope daily. Even if you do not have a high dollar phase contrast microscope, you can make valuable observations with they standard light microscope that is common to many high school classrooms. But what are you looking at when you observe samples at 100x, 400x, and even 1000x magnification? Today, I'll cover what you see when using a microscope. First, the "bugs" we note using the microscope are mostly single celled protozoa and a few multicellular lifeforms. The bacteria - or the actual workers - can be seen as part of the floc or as very, very small particles in the water. Some bacteria can become filamentous in form or are large enough to be seen with microscope, but most even at 1000x appear as small rods or spheres. We rely on the protozoa and higher life forms as indicator organisms for the underlying bacteria populations. For example, stalk ciliates are only present and active when there is sufficient dissolved oxygen and low levels of inhibitory or toxic compounds. This just happens to be where we are in decline phase growth or target F/M for most wastewater treatment plants. Multicellular forms such as rotifers, worms, or tardigrades appear even further along the F/M curve and can indicate old sludge or too low an F/M where you are carrying too much dead or inactive biomass. I recommend looking under the microscope daily for the following:
While I use the microscopic exam daily, I also like to run newer molecular testing that looks directly into the floc's microbial community. With high throughput sequencing, we look at DNA in the system and find our which microbes are present and at what % of total biomass reads (identifying DNA segments). This is a total microbial census of the MLSS and is good to run when establishing a baseline population database, making operational changes or on a quarterly basis for tracking long term changes. Following Microbial Community Analysis (MCA), we identify key microbes that are most important for good treatment. We can then use qPCR technology to track these specific microbes. qPCR is faster, highly quantitative, and cheaper than the full microbial census (MCA). Microbes consume dissolved oxygen (DO) when growing on wastewater pollutants. The rate at which the microbes consumer oxygen is the respiration rate. During periods of high loadings the respiration rates increases as the microbes become more active and cellular division rates increase. OUR rates increase in response to higher soluble organic loadings (BOD5), faster microbial division (low F/M), and following upset conditions when microbes are moving back towards stable population - this is log phase growth. Another facet of OUR testing includes a potential for a drop in respiration rates due to acute toxicity where previously respiring microbes are inactivated (killed). I have seen this with phenol, cyanide, and tall oil releases in industrial wastewater. So any large change in OUR rates should be investigated. If you are changing MLSS concentrations, you should standardize the OUR by using the SOUR calculation (divide OUR by MLSS or MLVSS in grams). Here is a link to the OUR test protocol that I have used for the past 25 years.
Being both deadly in enclosed spaces and a nuisance at even low levels, hydrogen sulfide is among the most problematic of compounds in wastewater treatment. Instead of covering the whole geochemical sulfur cycle, I want to look into the wastewater specific cycle that converts benign sulfates and sulfur into the problematic reduced sulfide species. Instead of graphics, I want to detail each form of sulfur found in wastewater.
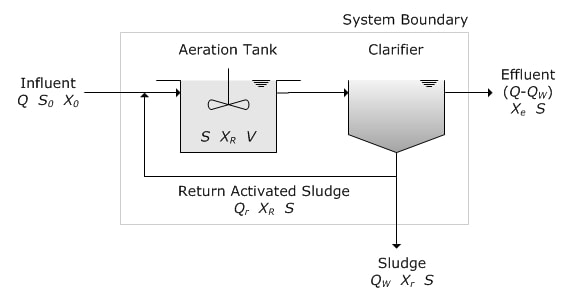
Activated sludge systems operate by moving a suspended biomass (MLSS) through an aerated basin and then separating solids for recycle back to the aeration basin. This process was invented in the early 20th century for treating wastewater and many variations on the process exist including contact stabilization, extended aeration, batch reactors, oxidation ditches, and pure oxygen systems. A key operating parameter has been the Food to Microorganisms (F/M) ratio. F/M is traditionally calculated using MLSS or MLVSS as the M and BOD5 as the F. Key is to be consistent and always know what you are using to calculate F/M and identify the best F/M ratio for operations.
Now we move to biofilm type systems versus suspended growth. Here we have tricking filters (old but still good), MBBR, and fixed film media. All relay on a biofilm which is actually just floc grown in attached form. Calculated F/M in this system is difficult since the biomass is attached to media. So we operate any biofilm based system on loading per unit of surface area - this can be BOD5/square meter or Ammonia/square meter. Again just be consistent in calculating. The key benefit of a biofilm vs suspended growth system is the ability to hold more biomass than comparable activated sludge units which rely totally on solids separation to keep biomass in the aeration tank. Of course this advantage is not present for all influents. For example, systems with significant influent oil & grease can foul (coat) the media which can be a big problem. |
AuthorErik Rumbaugh has been involved in biological waste treatment for over 20 years. He has worked with industrial and municipal wastewater facilities to ensure optimal performance of their treatment systems. He is a founder of Aster Bio (www.asterbio.com) specializing in biological waste treatment. Click to set custom HTML
Archives
April 2024
|
||||||




 RSS Feed
RSS Feed