 Spirochete from ucmp.berkeley.edu
Spirochete from ucmp.berkeley.edu Conditions to create septicity:
- Soluble organics (BOD5)
- Depleted free oxygen and nitrate - pushing to highly negative redox conditions
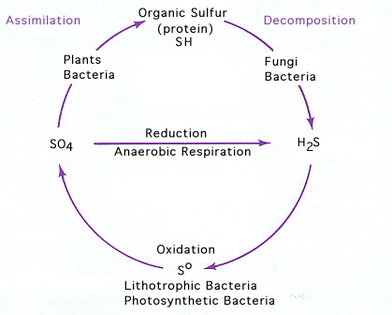
- Sulfate - is used as an electron acceptor under anaerobic conditions producing H2S
- Soluble organics become volatile organic acids that cause odors
- Dangerous H2S levels
- Odors
- Corrosion from H2S and sulfide oxidation
- Filament blooms from sulfides and short chain organic acids in wastewater plant influent
- Add aeration/mixing in EQ tanks
- Reduce primary clarifier residence time
- Discharge lifts-station more frequently (reduce collection system residence time)
- Pre-aeration before the treatment system
- Add nitrate to the collection system to increase redox and prevent true anaerobic activity

 RSS Feed
RSS Feed