- Avoid risk & costs of transporting contaminated soil off site
- If on an industrial site, activity can continue without disruption
- Effective in cleaning soil and not adding waste to RCRA landfills
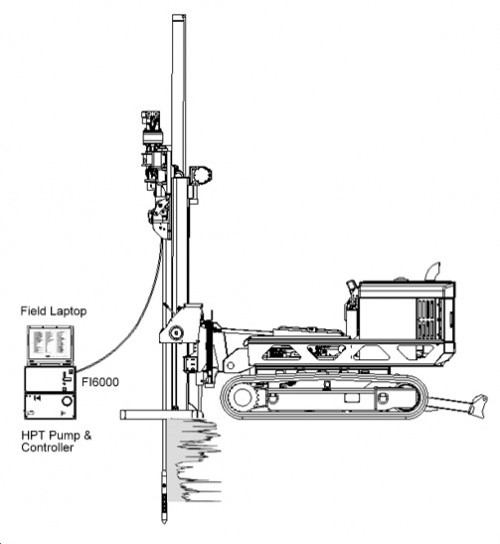
- High pressure injection into subsurface of a bioremediation "cocktail"
- Equipment and workers on-site only during injection events (Usually cleaned in 1 - 3 injection events)
- Works up to 40 feet depth and effective in most soils
- Workers inject a blend of oxygen release compounds (ORC), time release nutrients, and a microbial consortia (to rapidly increase the number of aerobic hydrocarbon degrading microbes in the subsurface).
- Injection points are done on a grid surrounding the contamination to ensure full remediation and build barriers to prevent off-site pollutant migration.



 RSS Feed
RSS Feed