To use India Ink with your microscope follow these steps:
- Pipette a small droplet of MLSS onto a glass slide
- Add an even smaller droplet of India Ink to the slide - just enough to color the water droplet dark gray/black.
- Place coverslip on the slide
- Observe using a standard microscope - you can use either regular light objective or phase contrast.
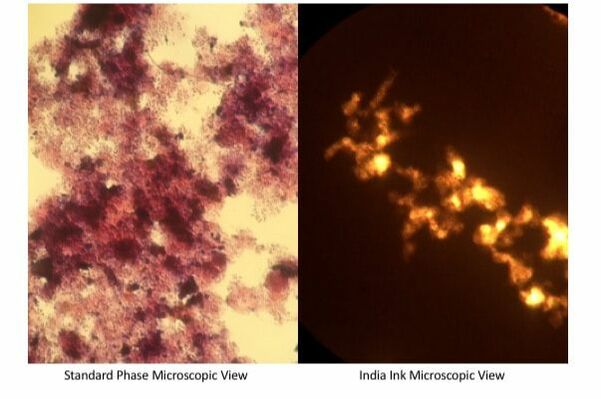
- The India Ink should move through the water and stain the slide. The clear zones with light shining through are floc with high levels of EPS that India Ink does not penetrate.
- Note the size and number of clear zones in the droplet.
- That is all that is required. I recommend doing India Ink tests at least 2x weekly and note any changes in EPS.



 RSS Feed
RSS Feed