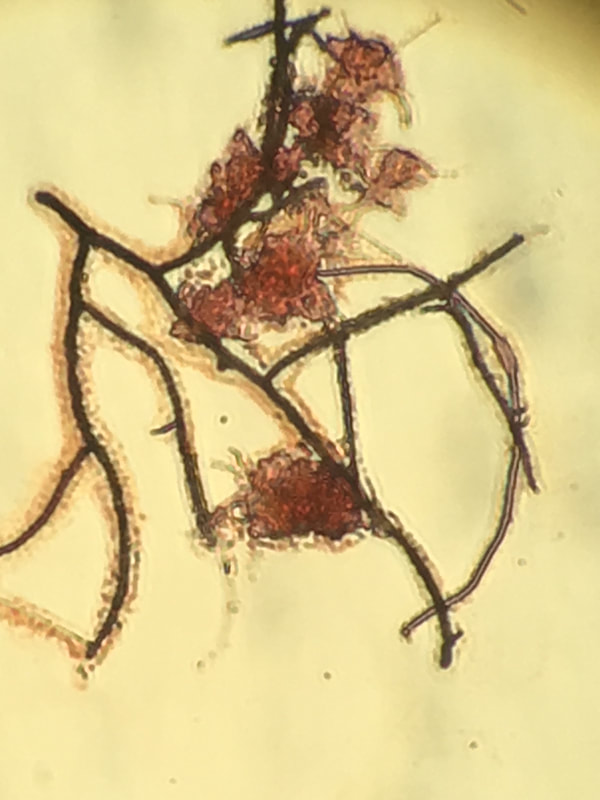
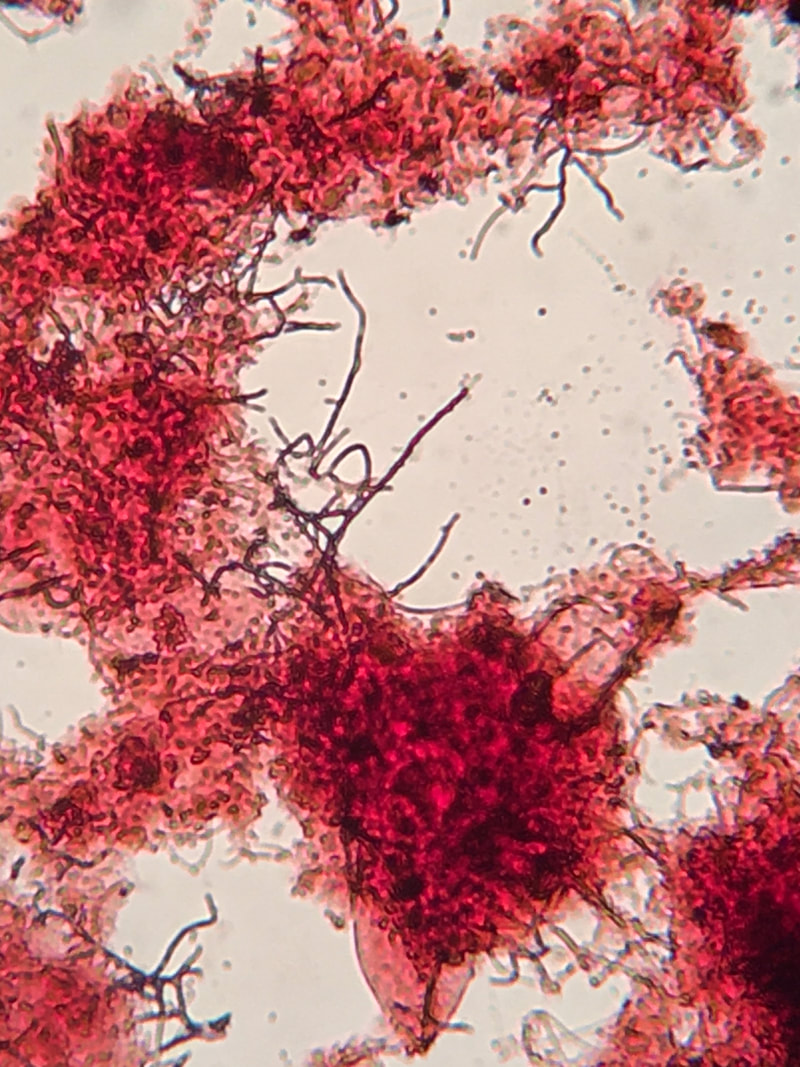
Gram staining is a common method to identify bacteria with a microscope. The stain differentiates bacteria into gram-positive or gram-negative based on their cell wall composition. Often used in medical or laboratory settings, it can also be useful in wastewater samples. You need to realize in wastewater with particulates and EPS, gram stain is often variable - so unless you have an non-floc organism such as Nocardia that stains strongly gram positive, you do not have large amounts of contrast.
Using a Gram Stain kit is easy. I'll walk through how I use it in wastewater samples.
- Pipette MLSS onto a slide - approximately 0.02 ml - you want enough to stain, but avoid big clumps of MLSS.
- Dry the slide for at least 20 minutes at room temperature or use a heat source to speed the process. Sample must be dry before starting the stain procedure.
- Organize the Gram Stain Solutions - (1) Gram Violet, (2) Gram Iodine, (3) Ethanol, and (4) Gram Safranin in order.
- Wear latex gloves to prevent staining your hands!
- Have a timer handy - once you start staining, time is important.
- Flood the slide with Gram Violet and allow to stain for 60 seconds.
- Rinse slide briefly with DI water.
- Flood the slide with Gram Iodine and allow to stain for 60 seconds.
- Rinse slide with DI water.
- Decolorize with the ethanol. Using a dropper, drip the ethanol over the slide drop by drop for 15 seconds. Key is to decolorize but not over-decolorize. You will get a feel for this as you perform more stains.
- Rinse with DI water.
- Flood slide with Gram Safranin and allow to stain for 60 seconds.
- Rinse with DI water.
- Allow slide to dry before examining under light microscope. Do not use the phase objectives.

 RSS Feed
RSS Feed