- Corrosion from hydrogen sulfide and acids
- Nuisance odors from volatile organic acids and hydrogen sulfide
- Potential worker exposure to hydrogen sulfide
- Organic acids and sulfides can trigger filamentous bulking in some activated sludge units
- Hydrogen peroxide
- Nitrate
- Ferric iron
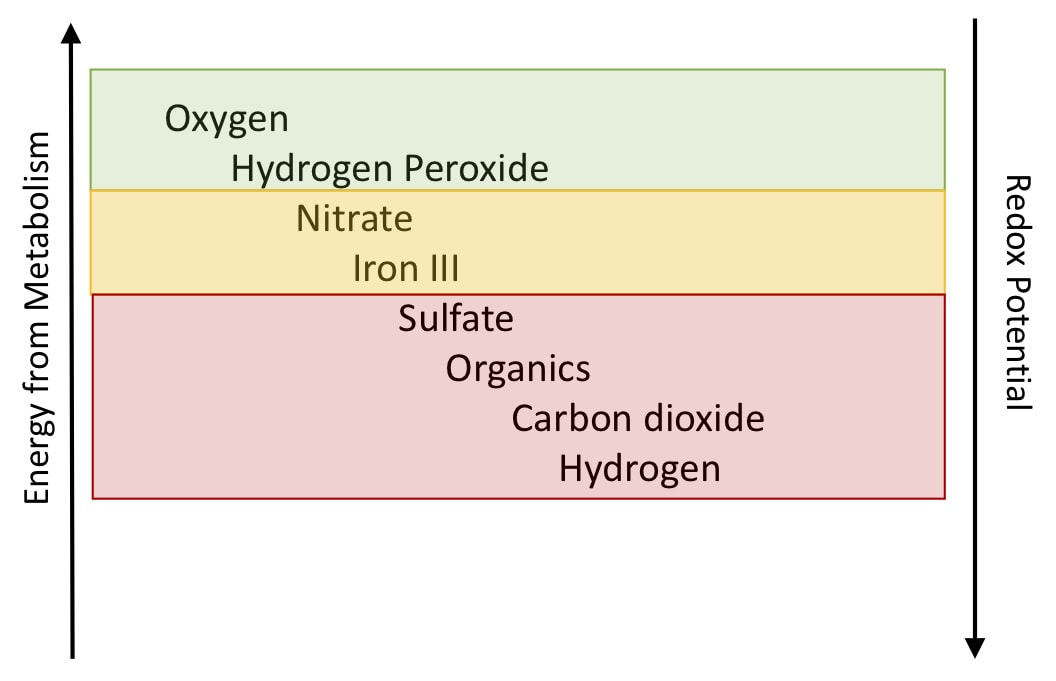
The different electron acceptors and different "levels" of anaerobic conditions are why I always ask for pH and redox information when doing odor control projects. As pH increases, sulfides and volatile organic acids remain more soluble and stay in the water column. This is why some odor control methods include adding caustic or other base solutions. ORP or Redox Potential gives us information on what electron acceptor is being used by microbes. At a pH of 7, when we are above -150 mV ORP - you will be using non-sulfate electron acceptors and odors will be minimal. Control strategies with ORP include adding peroxide, nitrate, or other electron accepting alternative.
With all the options, I like to pick a solution that best fits the system and problem. Do a system survey to see where problem originate and then think of various options. I have written on system using lift-station aeration systems (blowers), pure oxygen injection with nano-bubbles, nitrate solutions, and even targeted hydrogen peroxide additions. All have merits and ways to improve their effectiveness. The key is to not pick a solution until you do the survey.



 RSS Feed
RSS Feed