This example shows how volunteer labor, appropriate waste construction materials, and a bit of ingenuity can be used to treat wastewater. Now the final results may not be as high a water quality as waste treated in a modern wastewater treatment plant, it is a step in the right direction. I look forward to monitoring the Mumbai cleanup effort and see how well this innovative approach works.
|
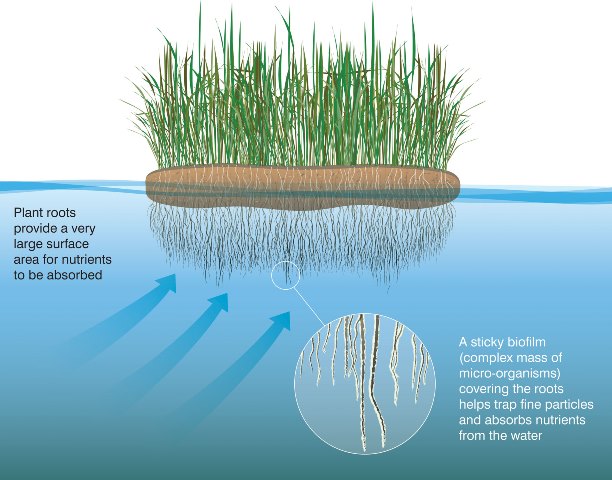
In a industry where we often discuss high cost treatment facilities with tanks, aeration, sludge handling, digesters, and even membranes; it is refreshing to see innovation done without the capital expense. According to an article in the Hindustan Times (link to article) In Mumbai there are several rivers polluted with raw domestic waste and stormwater runoff. The resulting pollution is 100x the target limits (the article was not clear, but am guessing they were discussion fecal coliform counts). To start cleanup, citizens and local academics are constructing artificial wetlands with used plastic drink bottles (that would have been trash) and plants that were being removed from local sites. Plant roots suspended in the river provide a surface for pollution degrading bacteria to colonize. The bacteria help the plants uptake nutrients from the water, while the beneficial root-associated microbes help degrade wastes and improve the water to a point where conditions do not favor enteric bacteria such as E. coli , bacterial pathogens, and parasites.
This example shows how volunteer labor, appropriate waste construction materials, and a bit of ingenuity can be used to treat wastewater. Now the final results may not be as high a water quality as waste treated in a modern wastewater treatment plant, it is a step in the right direction. I look forward to monitoring the Mumbai cleanup effort and see how well this innovative approach works. Nano oxygen bubbles - potential enhancement for high strength aerobic wastewater treatment5/23/2017
Many pollutants are most rapidly treated under aerobic conditions. When oxygen is not sufficient, ammonia oxidation often begins to fail as this biological reaction requires substantial amounts of oxygen. Even in moderate oxygen deficiency, you see reduction in bacterial floc density and growth of filamentous organisms having more surface area to access limited dissolved oxygen.
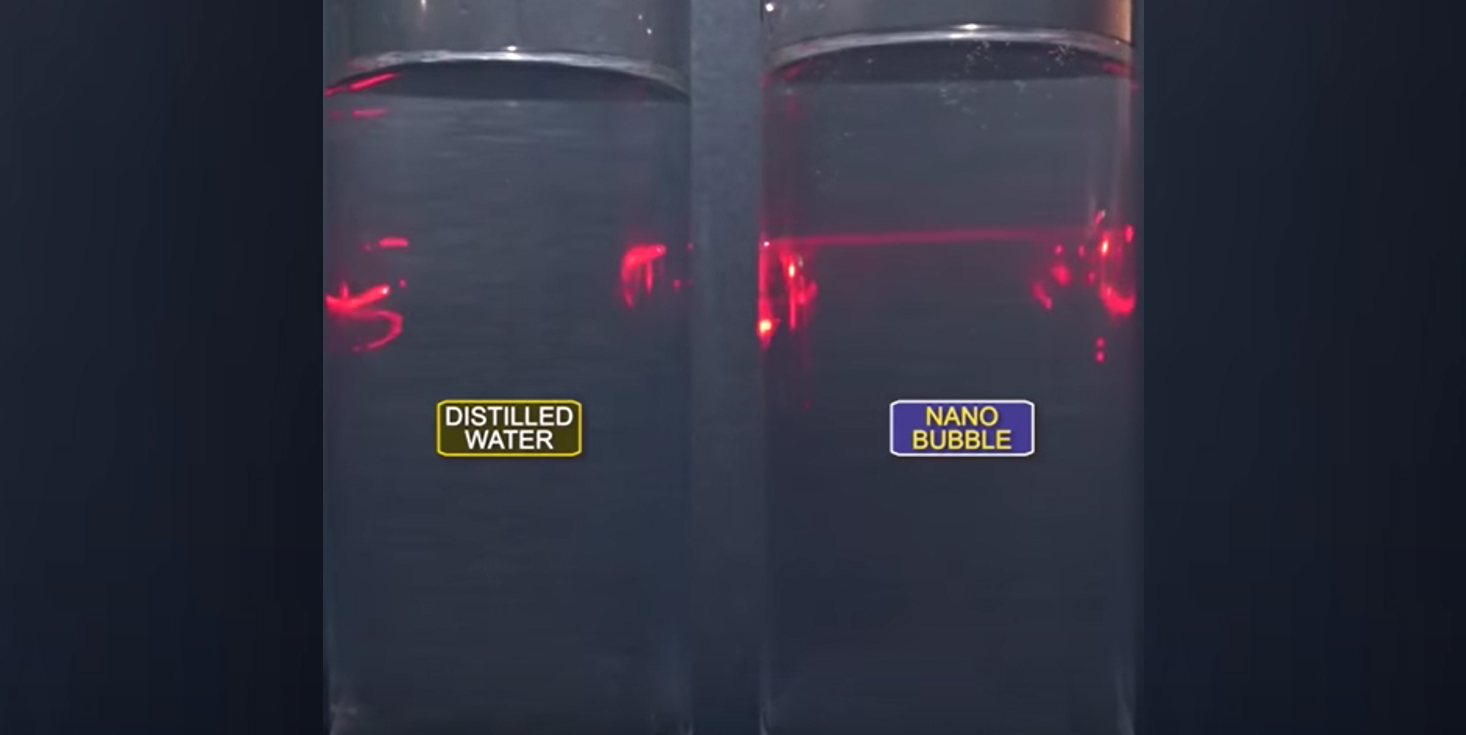
I have always been interested in how we have found low D.O. filaments in systems where a D.O. probe consistently reads at a minimum of 2.0 mg/L D.O. in the aeration basin. Using new genomic monitoring tools (16s rDNA tests), we have noted even obligate anaerobes in “aerobic” activated sludge plants. How can this be? We need to look at the floc or biofilm structure itself for the answer. Floc is composed of living microbes, extracellular polymers, adsorbed organics, and inorganics. It is not homogenous, instead being a highly diverse aggregate. Only the surfaces exposed to the water containing dissolved are experiencing abundant DO. As you move deeper into floc or biofilms, you find increasingly anoxic conditions. In older, denser flocs and mature biofilms, the deepest regions are anaerobic. The floc surface structure can also impact oxygen transfer from water into the underlying microbial layers. For example, in systems with inhibitory compounds, you find more EPS. This EPS protects microbial cells from toxic shock, but comes at the cost of efficient oxygen transfer. Other barriers for oxygen transfer are oils, grease, and non-volatile solids. Oxygen having poor solubility in water is part of the problem. Even in the finest bubble diffuser systems, oxygen transfer is relatively low. Even systems with pure oxygen injection, while operating at saturation conditions can have problems as the oxygen transfer to water is limited under atmospheric pressure. After hearing about new pressure adsorption systems where water becomes supersaturated with oxygen, I assumed that the excess oxygen would flash to the atmosphere as soon as pressure was reduced. Recently, I participated in a test of BlueInGreen SDOX (www.blueingreen.com) unit with pure oxygen in a lab setting. BlueInGreen’s SDOX uses pure oxygen transfer under high pressure to generate nano bubbles. Oxygen in nanobubble form has unusual properties like than seen with many other nano materials. In the test, dissolved oxygen was at 40 mg/L from the bench unit. What surprised me was that the D.O. remained above 30 mg/L for hours and above saturation for days. Why did the oxygen not flash off as I expected? A paper from Fernanda Yumi Ushikubo (University of Tokyo) “Evidence of the existence and the stability of nano-bubbles in water” gave me the answer. The research found that using air with 19% oxygen and 80% nitrogen did not make a stable supersaturated solution. When they used pure oxygen with the same pressure adsorption, the oxygen solution remained supersaturated for up to six days. The testing found that Zeta potential of pure oxygen bubbles was great enough for nano oxygen bubbles to repel each other preventing coalescing to larger bubbles. Air did not have the same magnitude of charge required to prevent coalescing. What does this mean for aerobic wastewater treatment? Pure oxygen nanobubbles have the potential to create stable supersaturated oxygen inside a biological treatment unit. The supersaturated solutions will enable oxygen to reach deeper into the floc/biofilm. This increases the number of microbes engaging in aerobic respiration and limits ecological pressures favoring low D.O. filament growth. The nano oxygen bubble technology could prove valuable for high strength industrial wastes and reduce both the required MLSS concentration and retention time. Using DNA testing of MLSS, we have found obligate anaerobic cultures present in system that maintain a D.O. between 1.5 and 2.5 at all times in the biological unit (aeration basin). How can this be possible?
Let's take a step back an look at the floc itself. In industrial system operating at a long sludge age bacterial floc has extracellular (EPS) materials and inorganics binding the bacterial matrix. If a long sludge age is coupled with quasi-toxic or inhibitory influent, the bacteria also excrete more EPS for protection. Key example of this is systems with periodic phenol and solvent loadings. The EPS acts as a barrier between the encased bacteria and water. While the water may have 2 mg/L dissolved oxygen, inside the floc you may have much lower oxygen residuals. As the EPS layer becomes more "fixed" not necessarily larger - the bacteria in the core region of the floc can be in true anaerobic conditions. This can spur the growth of obligate anaerobes that should not normally exist in the aerobic biological treatment unit. Another feature of this phenomenon is the system with high DO having low DO filaments. The oxygen barrier provided by EPS is also key to nutrient removal in systems utilizing anammox, aerobic granular sludge, and MBBR technologies. The removal of nitrate and nitrite from wastewater, also called denitrification, has become a required in wastewater treatment as we seek to reduce total nitrogen loadings into receiving waters. For years, wastewater treatment has included reduction in ammonia nitrogen - which is the most toxic form to aquatic life and has a very high oxygen demand. In ammonia oxidation, aerobic microbes transform ammonia into nitrite and finally nitrate (this is an aerobic reaction).
To remove both nitrate and nitrite from the water - thereby lowering total nitrogen discharge - a second anoxic (negative redox conditions) step with soluble organics present is required. Some early systems used added methanol and anoxic conditions to trigger bacteria into using nitrate/nitrite as an alternative to oxygen. More modern systems, have an anoxic stage where high soluble BOD influent and return MLSS (RAS) high in nitrate/nitrite mix. No matter which process is used, the anoxic conditions favor bacteria that can utilize nitrate and nitrite as an alternative electron acceptor when degrading soluble organics (BOD). Using the nitrate/nitrite in this manner produces di-nitrogen (N2) gas (and some more problematic NO gas). The N2 gas production is inside the floc - extracellular polymers and floc surface temporarily trap the small nitrogen bubbles. The gas acts to float the MLSS. Once floated, the nitrogen bubbles slowly emerge from the floc and the MLSS settles. Problems happen when denitrification occurs in secondary clarifiers causing floating sludge and TSS carryover to the effluent. Normally we can control secondary clarifier denitrification by increasing return rates (lowering residence time of the clarifier beds). Other control options include water spay and higher rake speeds to help the nitrogen bubbles escape the floc. Denitrification can also increase effluent TSS in polishing ponds and storm water ponds in cases where you have biological floc, nitrate/nitrite, and soluble organics (this can even be from biological solids decomposing in warm temperatures). Many industrial processes use sodium hydroxide (caustic) to neutralize acids or in other process areas. Once reaching target pH, engineers usually don't consider how monovalent cations (Na+) can impact the biological treatment unit.
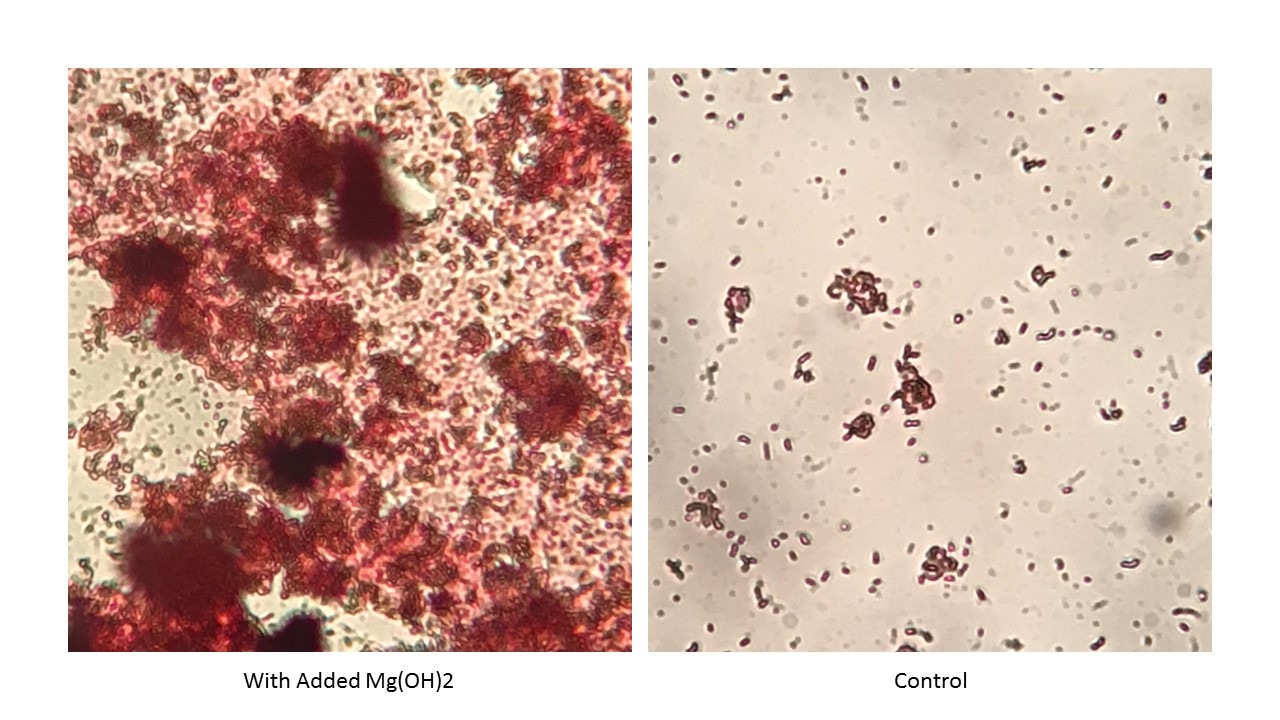
To understand the Na+ ion impact on floc formation, we need to look at floc composition. Floc is made up of living and dead microbes, extracellular polymers, adsorbed organics, and particulate inorganics. Microbial components including EPS have a slight anionic charge. This is why cationic polymers and metal salts are often used to coagulate and flocculate biological MLSS. Divalent and polyvalent cations neutralize charges and form needed links between cells and EPS. Charge neutralization with monovalent cations does not provide the "link" needed for biofilm or floc formation. Numerous studies have found floc formation problems with the ratio of Monovalent:Divalent cations increases above 2:1. Research including trivalent aluminum and iron came up with the following (M/D)/T < 1.0 with trivalent cations being measured in the solids fraction. A recent influent sample was not flocculating as expected in an activated sludge unit. Early correction attempts including adding bioaugmentation cultures were not giving improved flocculation. So, I ran a 48 hour test on biomass grown on the influent with and without added Mg(OH)2 (30 - 90 ppm as Mg). The photos above show the differences. The amount of biological solids did not significantly differ between the control and experimental flasks. But the amount of floc formation was visibly better with added Mg++ addition. So if see a change in floc formation/polymer usage concurrent with an increase in caustic use, you may want to look at the ratio of monovalent to divalent/trivalent cations in the system. Most engineers know that certain compounds such as PCBs, Dioxin, or DDT are not amenable to biological detoxification. Between non-biodegradable and readily-degradable compounds are a large spectrum of resistant or slowly-biodegradable compounds. When you mix multiple compounds on a single site, individual degradation rates are often different than single compound studies done in a lab. So what should be considered when evaluating compounds/sites for biological treatment:
Number 1 is based on the hazardous material itself - some compounds are not amenable to biological degradation. The next 4 questions cover environmental factors that can be altered to promote biological treatment or simplified - you must make the conditions favorable to microbial growth. Finally, Number 6 covers the existing microbial population. If the needed microbes are not present or in insufficient numbers, detoxification will not occur. This is where bioaugmentation with required cultures initiates biological treatment. |
AuthorErik Rumbaugh has been involved in biological waste treatment for over 20 years. He has worked with industrial and municipal wastewater facilities to ensure optimal performance of their treatment systems. He is a founder of Aster Bio (www.asterbio.com) specializing in biological waste treatment. Click to set custom HTML
Archives
April 2024
|


 RSS Feed
RSS Feed