To understand the Na+ ion impact on floc formation, we need to look at floc composition. Floc is made up of living and dead microbes, extracellular polymers, adsorbed organics, and particulate inorganics. Microbial components including EPS have a slight anionic charge. This is why cationic polymers and metal salts are often used to coagulate and flocculate biological MLSS. Divalent and polyvalent cations neutralize charges and form needed links between cells and EPS. Charge neutralization with monovalent cations does not provide the "link" needed for biofilm or floc formation.
Numerous studies have found floc formation problems with the ratio of Monovalent:Divalent cations increases above 2:1. Research including trivalent aluminum and iron came up with the following (M/D)/T < 1.0 with trivalent cations being measured in the solids fraction.
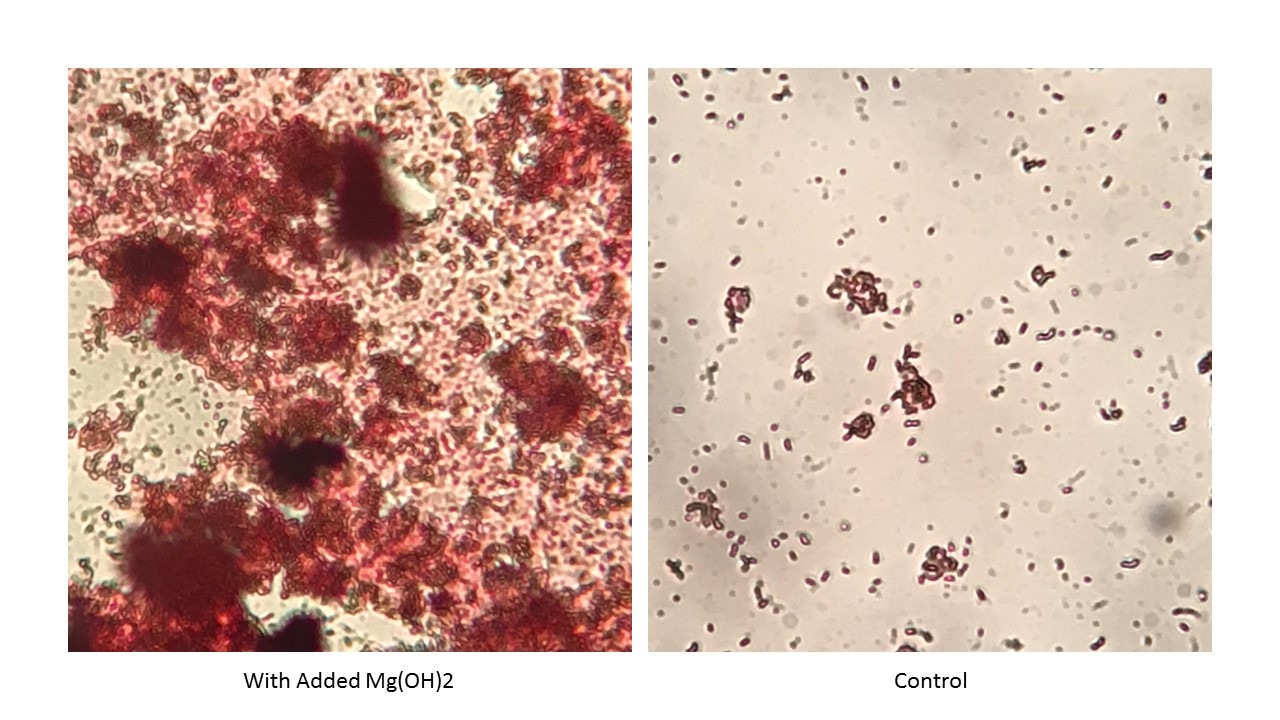
A recent influent sample was not flocculating as expected in an activated sludge unit. Early correction attempts including adding bioaugmentation cultures were not giving improved flocculation. So, I ran a 48 hour test on biomass grown on the influent with and without added Mg(OH)2 (30 - 90 ppm as Mg). The photos above show the differences. The amount of biological solids did not significantly differ between the control and experimental flasks. But the amount of floc formation was visibly better with added Mg++ addition.
So if see a change in floc formation/polymer usage concurrent with an increase in caustic use, you may want to look at the ratio of monovalent to divalent/trivalent cations in the system.

 RSS Feed
RSS Feed