I have always been interested in how we have found low D.O. filaments in systems where a D.O. probe consistently reads at a minimum of 2.0 mg/L D.O. in the aeration basin. Using new genomic monitoring tools (16s rDNA tests), we have noted even obligate anaerobes in “aerobic” activated sludge plants. How can this be? We need to look at the floc or biofilm structure itself for the answer. Floc is composed of living microbes, extracellular polymers, adsorbed organics, and inorganics. It is not homogenous, instead being a highly diverse aggregate. Only the surfaces exposed to the water containing dissolved are experiencing abundant DO. As you move deeper into floc or biofilms, you find increasingly anoxic conditions. In older, denser flocs and mature biofilms, the deepest regions are anaerobic.
The floc surface structure can also impact oxygen transfer from water into the underlying microbial layers. For example, in systems with inhibitory compounds, you find more EPS. This EPS protects microbial cells from toxic shock, but comes at the cost of efficient oxygen transfer. Other barriers for oxygen transfer are oils, grease, and non-volatile solids.
Oxygen having poor solubility in water is part of the problem. Even in the finest bubble diffuser systems, oxygen transfer is relatively low. Even systems with pure oxygen injection, while operating at saturation conditions can have problems as the oxygen transfer to water is limited under atmospheric pressure.
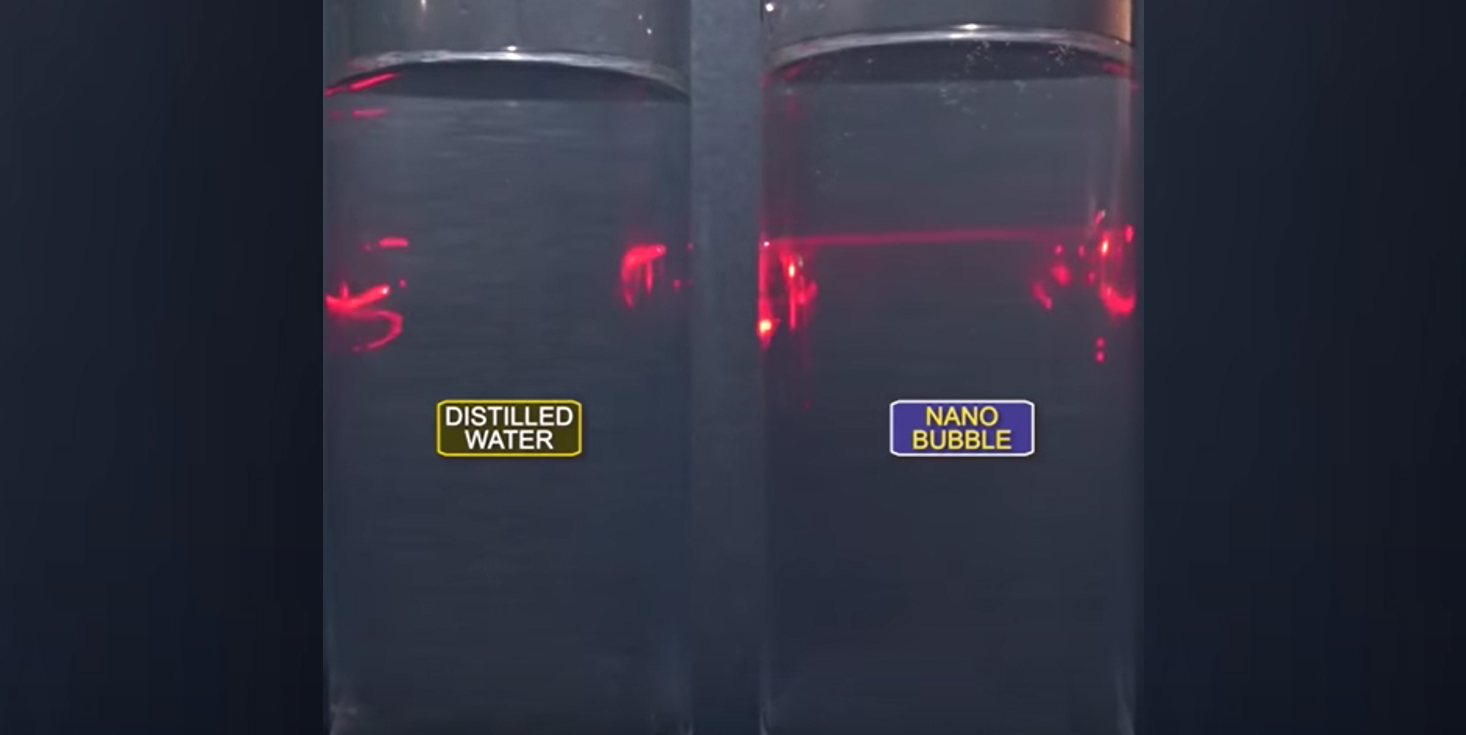
After hearing about new pressure adsorption systems where water becomes supersaturated with oxygen, I assumed that the excess oxygen would flash to the atmosphere as soon as pressure was reduced. Recently, I participated in a test of BlueInGreen SDOX (www.blueingreen.com) unit with pure oxygen in a lab setting. BlueInGreen’s SDOX uses pure oxygen transfer under high pressure to generate nano bubbles. Oxygen in nanobubble form has unusual properties like than seen with many other nano materials. In the test, dissolved oxygen was at 40 mg/L from the bench unit. What surprised me was that the D.O. remained above 30 mg/L for hours and above saturation for days.
Why did the oxygen not flash off as I expected? A paper from Fernanda Yumi Ushikubo (University of Tokyo) “Evidence of the existence and the stability of nano-bubbles in water” gave me the answer. The research found that using air with 19% oxygen and 80% nitrogen did not make a stable supersaturated solution. When they used pure oxygen with the same pressure adsorption, the oxygen solution remained supersaturated for up to six days. The testing found that Zeta potential of pure oxygen bubbles was great enough for nano oxygen bubbles to repel each other preventing coalescing to larger bubbles. Air did not have the same magnitude of charge required to prevent coalescing.
What does this mean for aerobic wastewater treatment? Pure oxygen nanobubbles have the potential to create stable supersaturated oxygen inside a biological treatment unit. The supersaturated solutions will enable oxygen to reach deeper into the floc/biofilm. This increases the number of microbes engaging in aerobic respiration and limits ecological pressures favoring low D.O. filament growth. The nano oxygen bubble technology could prove valuable for high strength industrial wastes and reduce both the required MLSS concentration and retention time.

 RSS Feed
RSS Feed