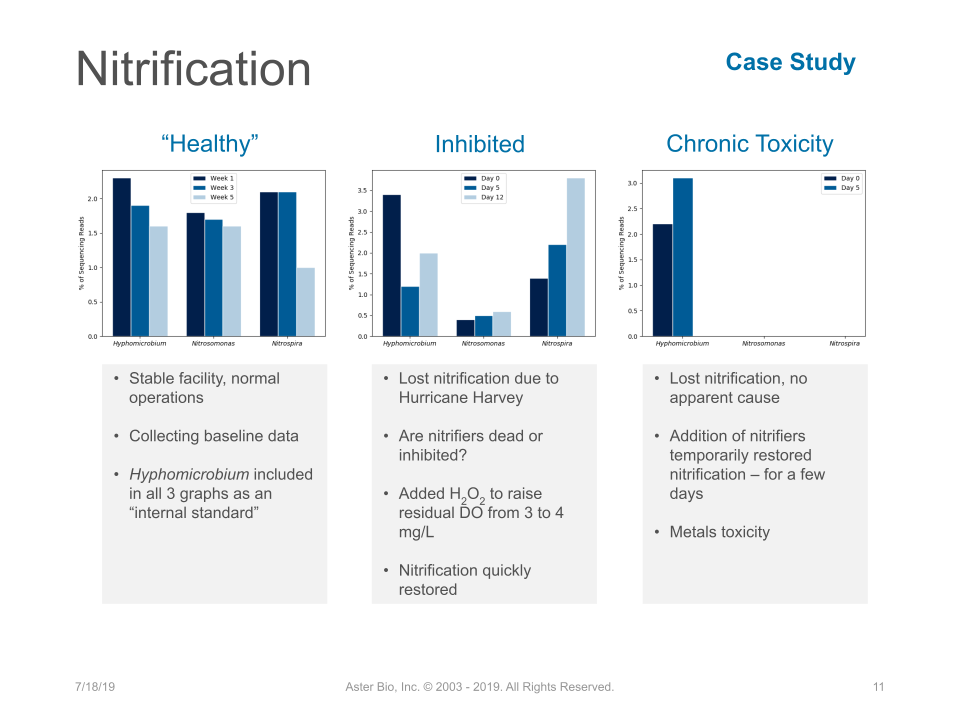
Step 1 - Determine what caused the problem by looking at influent & system data
- Acute toxicity event
- Gradual washout due to low DO, pH, or temperature slowing growth.
- Inhibition - temporary condition stops AOB/NOB metabolism - if it continues you will have washout.
Step 2 - Use qPCR test to confirm biomass AOB& NOB populations
Send a sample for a fast qPCR test for AOB & NOB checks. This gives information on what is causing loss of nitrification and the best corrective action. Given that a nitrifier panel using qPCR with wastewater specific primers is a rapid test and relatively low cost ($150 standard 2 day TNT or $250 rushed same day), this step can save money when compared to trucking in sludge or adding commercial nitrifiers. Based on quantitative results given by qPCR, you can chose the most efficient corrective action.
Step 3 - Corrective Action
- Make the environment favorable for AOB & NOB growth - pH, DO, Alkalinity, MCRT. Things that you can change.
- If part of the problem is inhibitory organics, adding heterotrophic cultures can help reduce some of the inhibition and get existing nitrifiers back to rapid growth.
- If qPCR finds insufficient AOB & NOB populations for ammonia (TKN) loadings, you can use either trucked in sludge or bioaugmentation with commercial nitrifiers. Dosing of commercial nitrifiers is done based on:
- Amount of ammonia and nitrite to be oxidized
- Environmental factors in the system - temp, pH, DO, etc
- How much time do you have for full recovery - catching problems early is very important

 RSS Feed
RSS Feed