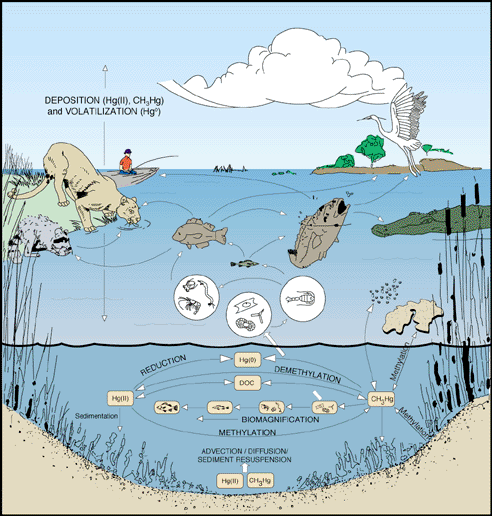
In the case of Muskrat Falls, construction of the dam builds a deep reservoir of water. At the reservoir bottom is organic debris and sediment that undergo slow anaerobic degradation. The reservoir also receives elemental and mercury II from natural sources such as the atmosphere (volcanoes, ocean volatilization) and underlying rocks. Additional mercury comes from human activities such as metal processing, mining, and mainly burning of coal. Most mercury inflow from the environment is in the "less" toxic but still toxic elemental form. When the dissolved mercury enters the anaerobic zone, microbes growing on the organic sediment convert elemental mercury into the more toxic methyl form that is more adsorbed by plants, fish, and all animals eating the fish. Adsorbed methyl mercury bioaccumulates and can cause mercury poisoning in populations depending upon contaminated animals as a food source.
It should also be noted that methyl mercury can be converted by UV radiation and aerobic microbial processes back into elemental form - which is part of the natural mercury cycle. It is the increased amount in mercury entering the cycle in an ecosystem that creates an abundance of methyl mercury.
Below is a great graphic of the mercury cycle from the USGS.

 RSS Feed
RSS Feed