If you smell something it is evaporating. In effect, something in the water is moving into the surrounding air and our noses are able to detect smells. Factors that impact evaporation rate include:
- Temperature
- Surface area
- pH & speciation
- Solubility
- Henry's Law (dissolved gas in a liquid is related to partial pressure)
- Sulfides (H2S) - classic rotten egg odor
- Organic acids - sharp rancid odors
- Mercaptans - RSH - putrid smell (detectable at extremely low concentrations)
- Dimethyl sulfide - cooked cabbage like odors
- Ammonia/urea - ammoniacal
Biochemistry Section
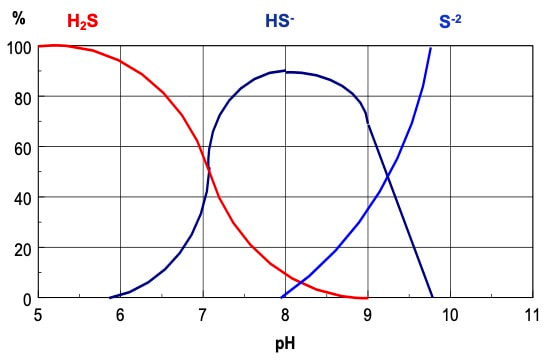
In wastewater, our odor causing problems are usually related to anaerobic biochemical reactions. We also need to consider speciation (or what form is present) of the compounds which is related to pH & ORP (redox). For example, H2S gas is the volatile form of sulfides in wastewater. As you decrease pH, you have increased % as H2S in the air versus S= (which is in the water).
In a typical collection system pH, bacteria thrive. Organics are present for oxidation. Bacteria then search for the most available, highest energy yielding reaction that acts as an ELECTRON ACCEPTOR. Once you deplete oxygen, you enter anoxic/anaerobic metabolism. For odors, the key is what electron acceptor the bacteria are using; as this will help determine redox, pH, and resulting odor causing compounds.
Control Options & How They Work
- Masking agents (fragrances) - fast control of odors but an after the fact solution.
- Sulfide binding chemistry - works on H2S but ignores other odor problems
- Oxidants - can directly oxidize odorous compounds or increase redox potential (provide a higher energy electron acceptor)
- Nitrate - provides a convenient, low-cost alternative electron acceptor to increase redox potentials.
- pH adjustment - increasing pH can reduce volatilization of sulfides and organic acids. However it can increase problems with ammonia odors.
- Biological treatment - this includes biological scrubbers and cultures added to lines. However, they do require oxygen or other higher energy electron acceptor to reduce odors.
No single odor control option fits every situation. If you have a consistent odor issue, you should investigate to determine what is causing the odors and work on the biochemistry of how and where these compounds are forming in the system. Once surveyed, you can take steps to prevent the formation of the underlying compounds by utilizing knowledge of biochemistry and speciation of volatile compounds. I have found success in using a combination of redox potentials (ORP), temperature, and pH to be highly effective in routine monitoring.

 RSS Feed
RSS Feed